PGx Report Explanation
See below for a sectional breakdown of Gravity's Pharmacogenetics Report

New Paragraph
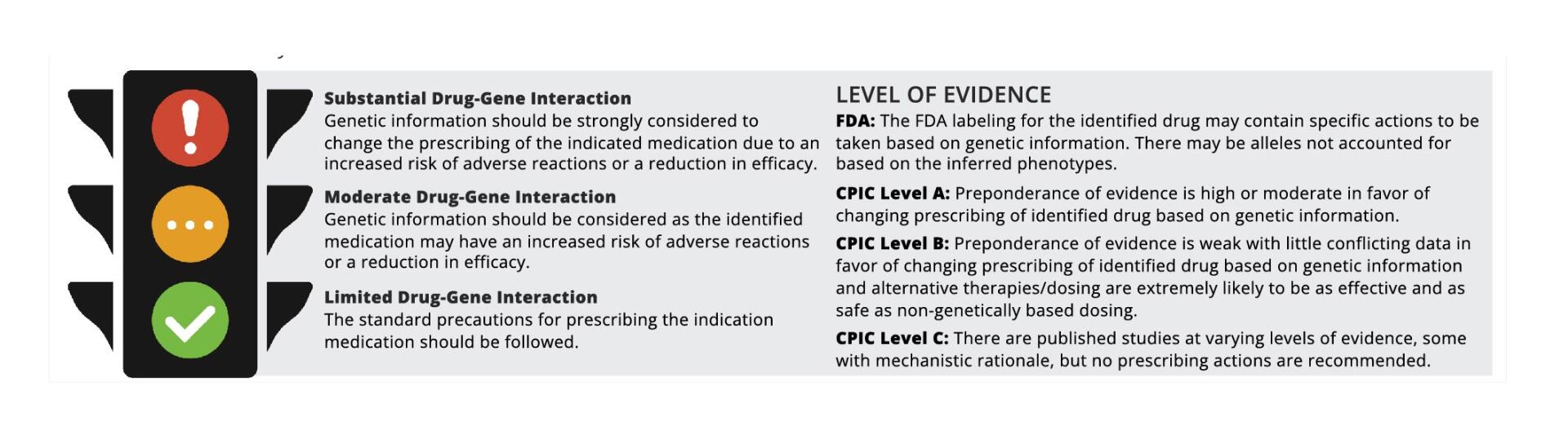
Section 1: Stoplight
This section of the report defines and describes the types of interactions between genes and drugs, as well as the level of evidence used to classify the strength of recommendations.
Throughout the report the following may be helpful:
CPIC-
Clinical
Pharmacogenetics
Implementation
Consortium
FDA- United States Food and Drug Administration
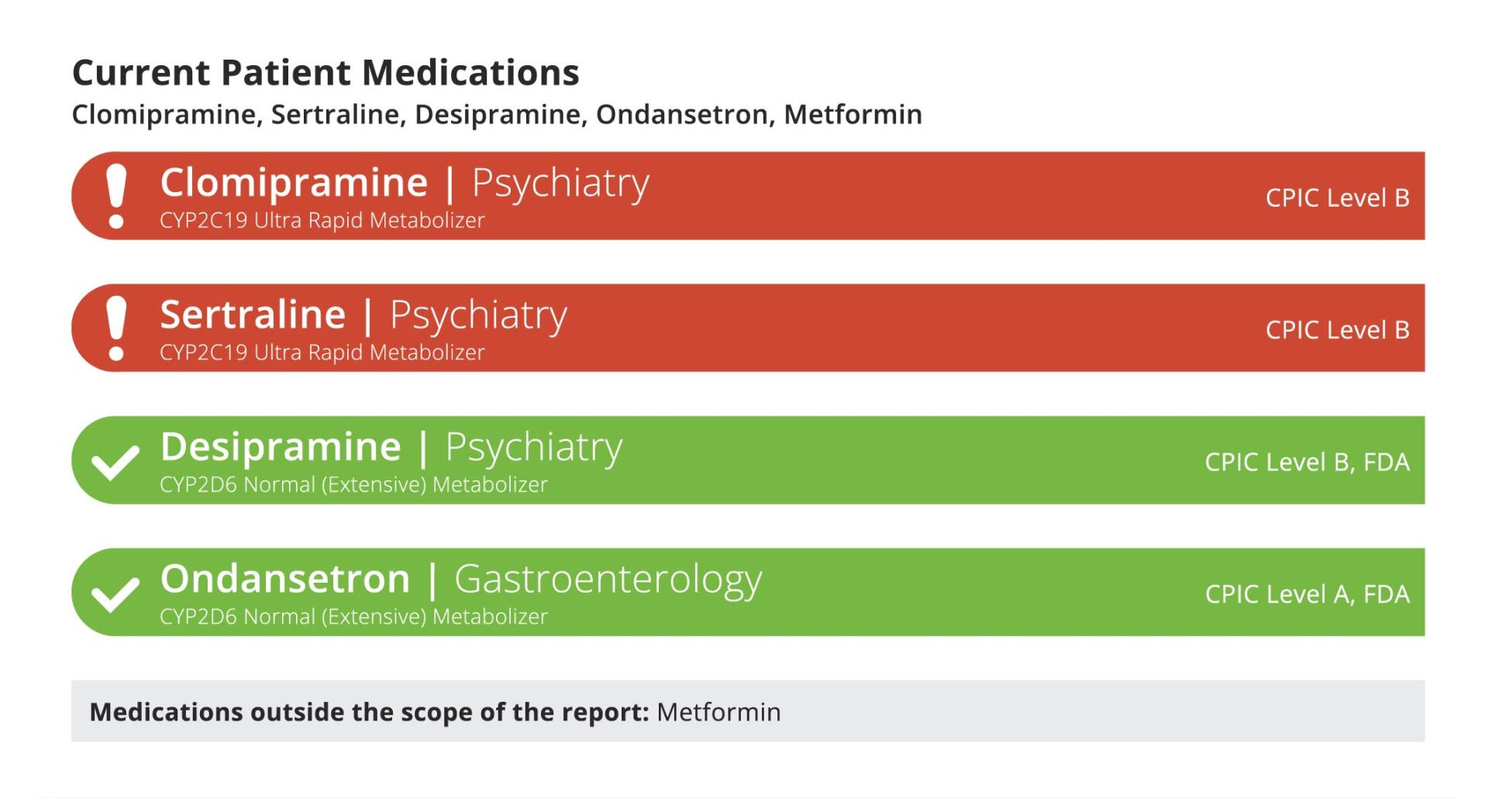
Section 2: Current Patient Medications
This section of the report highlights the current patient medications as provided by the physician.
A color coded system for the drug-gene interaction present is highlighted for the drugs included in this report:
Red = Substantial Drug-Gene Interaction
Yellow = Moderate Drug-Gene Interaction
Green = Limited Drug-Gene Interaction
Any indicated drugs that are not covered by this report will be indicated in the grey box reading “Medications outside the scope of the report”.
A drug that is covered by our PGx report, but does not have the corresponding gene ordered, will appear in green with the description “No Interactions Found”
- this means more information may be available for this drug if more genes are ordered.
Note: Medications appear on the report on their generic name, our algorithms make an effort to translate all brand names to their corresponding generic medications, however some discrepancies may exist.

New Paragraph
Section 3: Potentially Impacted Medications
This section of the report provides a summary table of all identified drug-gene interactions and their corresponding medications. This section is sorted first by therapeutic area (i.e. cardiovascular) then by drug class (i.e. statins).
A more detailed description of each drug-gene interaction can be found in the section below.
Section 4: Dosing Guidance
This section of the report describes specific dosing guidance for each included drug that has an abnormal result. Any drug(s) that have limited drug-gene interactions (green) will not appear here - it is assumed the standard dosing guidelines are to be followed.
In each drug-gene header you'll see the generic name of the drug as well as the therapeutic area it falls under. To the right is the CPIC level and/or the presence of pharmacogenetic information on FDA approved labelling.
The general guidelines we follow are CPIC level B/C and above or drugs with FDA labeling are considered to have a high enough level of evidence to include on our report. There are a few exceptions to this rule. The metabolizer status of the gene is listed as well as relevant implications and therapeutic recommendations.
The
Implications
and
Therapeutic Recommendations are pulled directly from CPIC guidelines or FDA approved drug labels. If a drug has a comment stating ”No recommendation based on ______ genotype.” it is because there is no high quality data provided by CPIC or the FDA, but there is an identified interaction between this gene and drug. If a drug-gene interaction as no recommendation based on genotype for both implications and therapeutic recommendations, they will appear in the table titled “The following medications have no recommended actions for the identified pharmacogenetic interaction.”


New Paragraph
Section 5: Test Details
This section of the report is composed of a summary table of diplotypes and/or genotype results for each gene and assay as applicable.
The genes that have a diplotype (i.e CYP2D6 *1*5) are calculated using an algorithm. Each are then converted into phenotype which describes the metabolizer status of each gene.
WANT THE FULL SAMPLE REPORT?
We promise to only send you the good stuff. No spam.
We will get back to you as soon as possible
Please try again later



